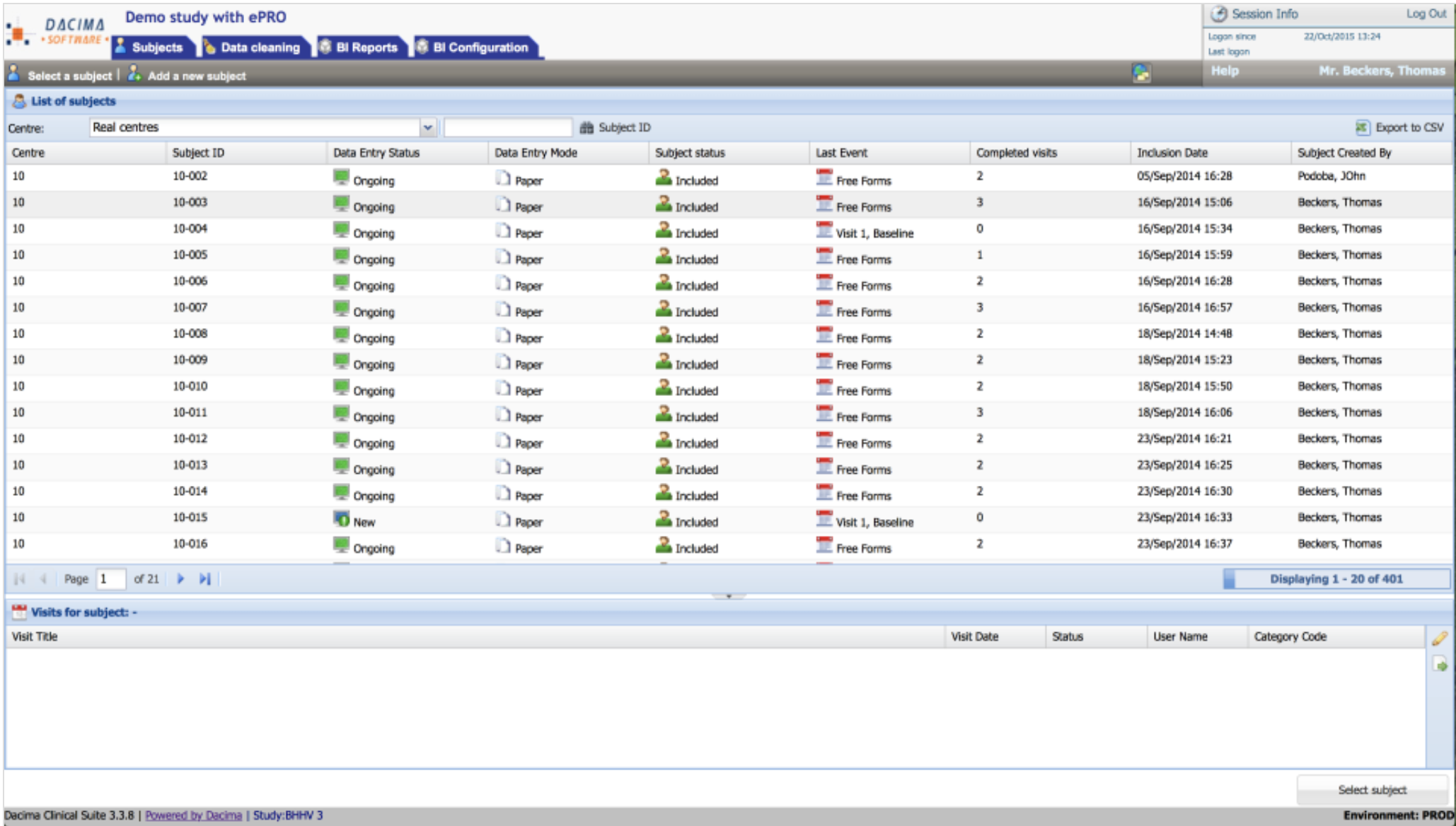
Dacima Clinical Suite
About Dacima Clinical Suite
Most Helpful Reviews for Dacima Clinical Suite
1 - 5 of 5 Reviews
Aaron
Used free trial
OVERALL RATING:
5
EASE OF USE
5
CUSTOMER SUPPORT
5
Reviewed March 2016
A fully featured, excellent, affordable software system that is Title 21 CFR Part 11 Compliant.
We have been using Dacima for a clinical trial, and needed a full audit trail to comply with FDA guidance for clinical trials. Dacima is a small Canadian company, and it has been a pleasure to work with them on our trial. Their system is most appropriate for longitudinal studies, as it can fully integrate a range of study components longitudinally. Our trial with Dacima is fully integrated into their system, including automated text (SMS) reminders for study visits, electronic consents, electronic CRF, enrollment, randomization procedure, and participant surveys. Dacima's services were able to fit within an NIH budget for our clinical trial, and they have provided our team at Emory with a fully featured system that meets all our need for the trial. They also have been very responsive in developing functionality particular to our study. I think this is in part because the CEO has a PhD in Epidemiology, so he understands the needs of academic institutions in implementing clinical trials. In sum, I highly recommend Dacima.
Vendor Response
Thanks so much for this review! We are proud to work with you on your project! The Dacima Team
Replied March 2016
G.
Used free trial
OVERALL RATING:
5
EASE OF USE
5
CUSTOMER SUPPORT
5
Reviewed March 2016
Control, ease of use, cost -- DACIMA provided a one-stop solution for our clinical data collection
Pros: - Control of all aspects of the study / registry from your own offices - Cost is very low compared to other customized solutions - Easy to make edits and duplicate forms - When needed, programmer support is quick and excellent - Wide range of variable types and dependencies - Lots of user control and permission settings - We've had good feedback from doctors that the interface and forms looked very professional - Instant compliance with required data management requirements Cons: - Technical support for some changes (although DACIMA makes them < 24 hours) - Saved changes make a "jump" to the top of the page instead of keeping you where you were in the form
Vendor Response
Thank you for your review! We appreciate and value your response very much. The Dacima Team
Replied March 2016
Elizabeth
Used free trial
OVERALL RATING:
5
EASE OF USE
5
CUSTOMER SUPPORT
5
Reviewed May 2016
Sophisticated eCRF design software, easy to use but has multiple functions for any type of study.
I worked with President and CEO, John Podoba on designing a eCRF for my study. I was a beginner and it did not take long for me to pick up the subtle functions of the software. John provided excellent customer service and took the time to make sure that I had all the information I needed to create a eCRF that was intuitive, streamlined and robust. I imagine that Dacima will make it's mark and become a leader in the Clinical Data Managment Software market.
Mira
Used free trial
OVERALL RATING:
5
EASE OF USE
5
CUSTOMER SUPPORT
5
Reviewed August 2015
Great EDC
Dacima offered us a solution tailored to our unique needs, which included limited internet access, inexperienced data entry personnel, a bilingual interface, project-specific data reporting forms and security requirements. Dacima has provided outstanding service. I give Dacima Software my highest recommendation and sincere admiration for its professionalism and dedication to providing work of the highest quality.
Rabie
Used free trial
OVERALL RATING:
5
EASE OF USE
5
CUSTOMER SUPPORT
5
Reviewed March 2016
DACIMA is a powerful and friendly datamanagement software
I've been using the software for about 3 years till now, and it helped my team to build up very powerful eCRFs. Our clinical studies became easier to follow. In the other hand, using the DACIMA Software helped the investigators to be more efficient at the patient enrollment and monitoring. The alert procedures are very supportive. Moreover, our datamanager is closely assisted in forms building.
