Clinion EDC
About Clinion EDC
Clinion EDC Pricing
Please contact Clinion IT Services for pricing details. sales@clinion.com
Starting price:
$1,000.00 per month
Free trial:
Available
Free version:
Not Available
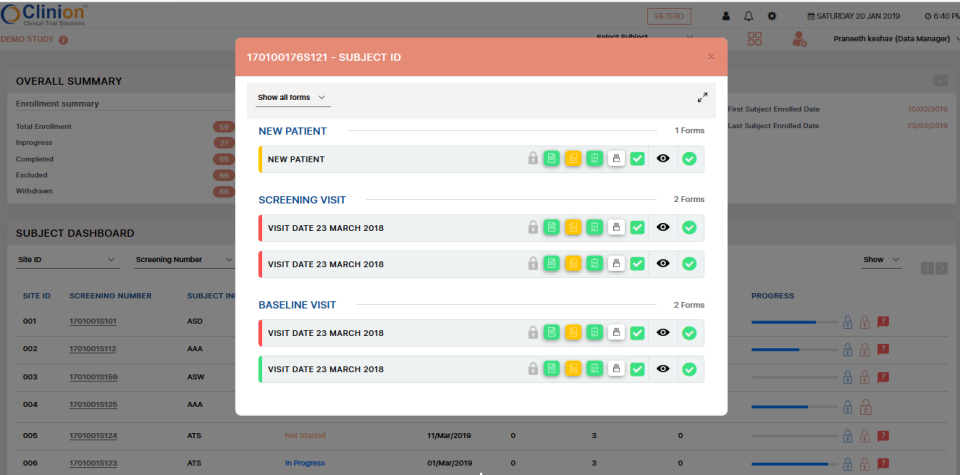
Most Helpful Reviews for Clinion EDC
1 - 5 of 14 Reviews
Swapnali
Research, 51-200 employees
Used daily for more than 2 years
OVERALL RATING:
5
EASE OF USE
4
VALUE FOR MONEY
5
CUSTOMER SUPPORT
5
FUNCTIONALITY
4
Reviewed February 2022
Excellent Software to use
Wonderful experience with Team Clinion
PROSWe (Target Institute of Medical Education and Research) are using Clinion EDC platform since 2015. We were absolutely new to this EDC but Clinion team made it easy for all of us. Clinion team helped us to shift from the conventional paper version of Case Record Form to Electronic version. EDC platform is very useful for our institute as it is very fast, efficient, environment friendly with high security. It also helped our institute in improving data quality, data collection, validation, and processing. Clinion EDC paltform data is reported helps us to report data in real-time, ensures data is readily available for central review.Clinion team is always ready to resolve all our concerns and queries. Clinion Team made use of EDC very easy for all of us.
CONSWe are using EDC platform for our majority of clinical trials and there is nothing to complain about software and Team Clinion. They are always ready to solve our issues and with new technology, they are fulfilling our requirements.
Ravi Shanker
Research, 201-500 employees
Used daily for more than 2 years
OVERALL RATING:
5
EASE OF USE
5
VALUE FOR MONEY
5
CUSTOMER SUPPORT
5
FUNCTIONALITY
5
Reviewed February 2022
Clinion is a 21 CFR Part 11 validated Electronic Data Capture and CDMS
Integrated IWRS with CDMS. real time email alert on SAE occurrence.
PROSuser friendly system, easy navigation, customizable report. customer friendly support/ helpdesk team
CONSDashboard report to be upgraded. summary generated report to be also customizable
Nikunj
Research, 51-200 employees
Used daily for less than 12 months
OVERALL RATING:
2
EASE OF USE
2
VALUE FOR MONEY
4
CUSTOMER SUPPORT
3
FUNCTIONALITY
1
Reviewed November 2019
CBCC Global Research review
Overall good product, the performance and functionalities of the product can be further enhanced, and bench marked with leading product available in the market.
PROSOn-line design testing- As if the testing/checking of the eCRF and edit checks can be performed immediately after publishing it. Navigation is quite easy.
CONSApplication behavior and designing of the edit checks- particularly about edit checks when it gets misfired. Data Validation is restricted only for the new data i.e. edit checks will be run on the newly entered data only. Sometimes, the application gets suddenly log-off due to unknown reasons.
zenith
Research, 11-50 employees
Used daily for more than 2 years
OVERALL RATING:
5
EASE OF USE
4
VALUE FOR MONEY
4
CUSTOMER SUPPORT
4
FUNCTIONALITY
4
Reviewed November 2022
Recommended EDC for clinical Trail data
Overall good experience and recommended to others.
PROSUser friendly, turn around time of support team
CONSFew limitations related to features and vertical panel programming
Reason for choosing Clinion EDC
Features, support, cost effectiveness
Vendor Response
Thank you, Zenith!
Replied December 2022
Venkata Ramana
Biotechnology, 10,000+ employees
Used daily for more than 2 years
OVERALL RATING:
5
EASE OF USE
5
VALUE FOR MONEY
5
CUSTOMER SUPPORT
5
FUNCTIONALITY
5
Reviewed November 2022
Clinion EDC review
Overall ease of use and efficiency
PROSEasy availability of data, rapid formal reporting , faster accumulation and data transfer.
CONSClinion EDC covers all the aspects of data capture
