Find the best Clinical Trial Management Software
Compare Products
Showing 1 - 20 of 83 products
Sort by
Reviews: Sorts listings by the number of user reviews we have published, greatest to least.
Sponsored: Sorts listings by software vendors running active bidding campaigns, from the highest to lowest bid. Vendors who have paid for placement have a ‘Visit Website’ button, whereas unpaid vendors have a ‘Learn More’ button.
Avg Rating: Sorts listings by overall star rating based on user reviews, highest to lowest.
A to Z: Sorts listings by product name from A to Z.
Complion
Complion
Designed for hospitals, medical centers, trial sites and more, Complion is a cloud-based clinical trial management solution that helps handle manual processes on a centralized interface. The software automates investigator regulat...Read more about Complion
RealTime-CTMS
RealTime-CTMS
RealTime-CTMS is a web-based clinical trial management system. It is designed for sponsors, CROs, research sites, university medical centers, plus other research-based organizations. To help with overall study operations, from set...Read more about RealTime-CTMS
ClinCapture
ClinCapture
Captivate EDC is a cloud-based electronic data capture solution that assists medical researchers and practitioners with data capture and forms management. Key features include WYSIWYG form editor, risk-based monitoring, activity d...Read more about ClinCapture
Visual Planning
Visual Planning
Visual Planning is a hybrid resource management and scheduling platform that helps businesses to manage their assets and day-to-day operations. The solution can be deployed on-premise or hosted in the cloud. Visual Planning o...Read more about Visual Planning
ClinPlus CTMS
ClinPlus CTMS
ClinPlus CTMS by Anju Software is a clinical trial management system (CTMS) designed to help sponsors and contract research organizations manage clinical trials. This solution offers workflow tools to help reduce outdated processe...Read more about ClinPlus CTMS
CRFweb
CRFweb
Clindox's integrated EDC application, CRFWEB, is the perfect tool for your clinical research needs. It offers eCRF, eDiary, and ePRO functionality plus integrated Randomization and MedDRA coding modules. With this software, you ...Read more about CRFweb
Castor ePro
Castor ePro
Castor ePro is an online platform that enables clinical trial teams to create, send, and track patient-reported outcomes (PRO) surveys electronically. Castor ePro makes it easier for clinicians to collect data from patients in rea...Read more about Castor ePro
OpenClinica
OpenClinica
OpenClinica is a clinical data management and electronic data capture solution designed to help medical institutions capture electronic data and streamline clinical trials using electronic case report forms (eCRFs) and a drag-and-...Read more about OpenClinica
Smartsheet
Smartsheet
Smartsheet is a work execution platform and collaboration tool with a familiar spreadsheet-like interface that helps teams plan, track, and manage projects in real-time. Smartsheet features include a range of project management to...Read more about Smartsheet
Encapsia
Encapsia
Encapsia is a clinical data suite designed to help organizations streamline capturing, management, and reporting of electronic data in clinical trials. It includes an eSource app, which allows users to gather, validate, and clean ...Read more about Encapsia
Ripple
Ripple
Ripple is a web-based solution designed to manage recruitment and post-enrollment tracking of clinical trial patients. It can be used by academic institutions, contract research organizations, small medical device companies, plus ...Read more about Ripple
Dacima Clinical Suite
Dacima Clinical Suite
Dacima Clinical Suite is a cloud-based electronic data capture (EDC) and study management (CTMS) system designed to streamline clinical trial data collection processes. This solution includes interactive dashboards, a CDISC-compli...Read more about Dacima Clinical Suite
Veeva Vault
Veeva Vault
Veeva Vault is a cloud-based content management solution with built-in collaboration features designed specifically for the life sciences industry. It comprises multiple modules that independently perform separate functions. ...Read more about Veeva Vault
Clinical Conductor CTMS
Clinical Conductor CTMS
Clinical Conductor CTMS (CC CTMS) is the leading clinical research management solution, utilized by more sites in the world than any other CTMS. Hospitals, hospital networks, health systems, large site networks, and even smaller ...Read more about Clinical Conductor CTMS
Track.health
Track.health
Track.health helps businesses in the healthcare industry streamline data acquisition for clinical study or trials. It captures EDC and eCOA data on a centralized platform. Track.health allows professionals to implement remote...Read more about Track.health
ClinicDr
ClinicDr
ClinicDr is a cloud-based medical practice management software which helps chiropractors handle treatment documentation, billing services, credentialing, claim scrubbing, scheduling, appointment reminders, telemedicine and more. K...Read more about ClinicDr
iMednet
iMednet
iMednet is a cloud-based electronic data capture platform designed to help the healthcare industry capture, clean, adjudicate and manage data across clinical trials. The application enables research teams to build and implement st...Read more about iMednet
MainEDC
MainEDC
Complex eClinical Solution for EDC/ERT/eCOA that significantly speeds up the start of clinical trials (up to 5 days), saves budget (up to 80% for monitoring), increases the capitalization and attractiveness of your company (noted ...Read more about MainEDC
Prelude EDC
Prelude EDC
Designed for clinical research, Prelude EDC is a CFR, Annex 11, and HIPAA compliant EDC solution that streamlines the data collection process, automates data cleaning efforts, and monitors the state of electronic case reports form...Read more about Prelude EDC
Google Cloud
Google Cloud
Featuring G-Suite and GCP, Google Cloud is a platform that provides a reliable and easy-to-use set of solutions that can be used to tackle the toughest challenges in any type of industry. It provides secure storage options, integr...Read more about Google Cloud
Popular Comparisons
Buyers Guide
Last Updated: March 16, 2023Clinical research organizations (CROs) face several challenges, such as conducting multiple trials simultaneously, adhering to regulatory compliance, and ensuring transparency in financial transactions. To deal with these challenges, CROs need to be proactive, and clinical trial management software helps them do that. The software assists CROs and other healthcare organizations in protecting patients’ health and financial data to avoid compliance issues.
There are various clinical trial management software applications available on the market. However, choosing software with features that are right for your organization can be time-consuming. To help you, we've created this buyers guide with the information you need to know when making a purchase decision.
Here's what we'll cover:
What is clinical trial management software?
Clinical trial management software is used to manage clinical trials done in clinical research. It serves as a single, centralized repository to store all the clinical research studies conducted in a CRO.
The software helps CROs streamline daily tasks, such as team collaboration, performance management, tracking of deadlines, and regulatory document management. It also assists in planning, reporting, and benchmarking clinical research processes.
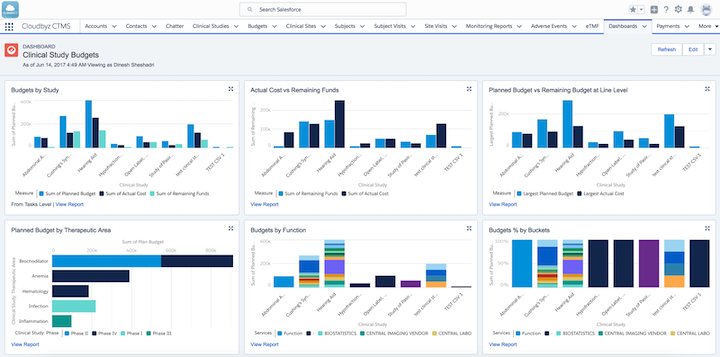
Clinical study budget analysis in Cloudbyz clinical trial management software (Source)
Common features of clinical trial management software
Different clinical trial management software vendors' features offerings will vary. Selecting software with the right features for you is easier when you know what the most common features are, and what they do.
Enrollment management | Allows users to plan and enroll targets (number of required patients) for different clinical trials. |
Document management | Allows users to create, manage, store, and track electronic documents within a single database. |
Patient database | Allows users to store patient data to run specific searches within the database based on multiple criteria, such as age, gender, body mass index (BMI), medical condition, and prescribed medication. |
Patient scheduling | Allows users to schedule patient visits for specific clinical trials that match the patient's profile. |
Compliance management | Allows users to ensure compliance with regulatory requirements, such as 21 CFR Part 11 and HIPAA regulations. |
Financial management | Allows users to track payments, maintain invoices, and manage accounts payable and receivable. |
Reporting | Allows users to generate different reports to convert data into actionable tasks in real time. |
Third-party integration support | Allows users to integrate the software with other existing software solutions, such as electronic medical records (EMR), for smooth exchange of patient health data. |
What type of buyer are you?
Before you start evaluating software options, you'll want to know which buyer category you belong to, so you can understand what to look for in a solution. Most buyers fall into one of the below categories.
Small and midsize CROs (5-100 employees): These CROs are usually founded by individuals or groups of physicians/scientists with expertise in a specific field. They have fewer employees, focus on a niche area of research, and usually operate out of a single location. Such buyers should consider software with features that are aligned with their area of clinical research or specialization. Some of the common software features they should consider are enrollment management, document management, patient database, and patient scheduling.
Large CROs (more than 100 employees): These CROs have a larger number of employees and several clinical research trials and studies on their annual agendas. They have to manage large volumes of data—documents, contracts, and financial transactions—for all the patients involved in trials. They also have to take care of legal and compliance requirements for clients, mostly big pharma companies. Such buyers should opt for a complete clinical trial management software suite that offers features such as compliance management, financial management, reporting, and third-party integration.
Benefits of clinical trial management software
While some of the benefits of clinical trial management software may already be evident, we've listed the most notable ones in this section.
Ensuring compliance: Large volumes of data (patient data, billing information, study designs, deadlines, etc.) are processed during clinical trials. The software helps streamline the clinical audit process by tracking, managing, and consolidating all data into a single, centralized system. It also consolidates data from previous internal and external audits, compliance certificates, and other related processes. Further, it tracks any process deviations in daily protocols.
Tracking financial data: Clinical trial management software ensures accurate invoicing for all stakeholders and timely payment for all research trials and studies. It also lets CROs monitor their bottom lines to help control costs and filter out trials that aren’t bringing in revenue.
Managing data: The software offers a single and secure location for data collection, storage, and retrieval. Information—such as patient data or clinical study-related documents—is stored in a centralized repository and accessed by all authorized personnel through a searchable library.
Key considerations when selecting clinical trial management software
Listed below are some important points to consider before you purchase clinical trial management software.
Mobile app availability: The software you’re planning to purchase should be available as a mobile app on iOS and Android smartphones or tablets. Mobile-ready software will allow you to perform tasks such as enrolling patients, updating contact details, and signing documents digitally, even when you’re on the move.
Customer support: Check out the type of support offered by your shortlisted vendors: 24/7, 24/5, or only during business hours. Also, ask about the available support channels, such as email, phone, or live chat. Support services will help you fix software issues that can hamper productivity or result in downtime.
Market trend to understand
Virtual clinical trials: New technologies, such as wearable sensors, smartphone apps, and mobile health (mHealth), are paving the way for virtual clinical trials. In virtual clinical trials, all steps, including patient enrollment, data collection, and follow-ups, are executed through video or audio calls. Such trials are likely to help reduce patient enrollment time and result in a more diverse pool of participants. Pharma companies across the globe are increasingly collaborating with major players in the clinical care space to conduct virtual trials.
Note: The application selected in this article is an example to show a feature in context and isn’t intended as an endorsement or recommendation. It has been obtained from sources believed to be reliable at the time of publication.